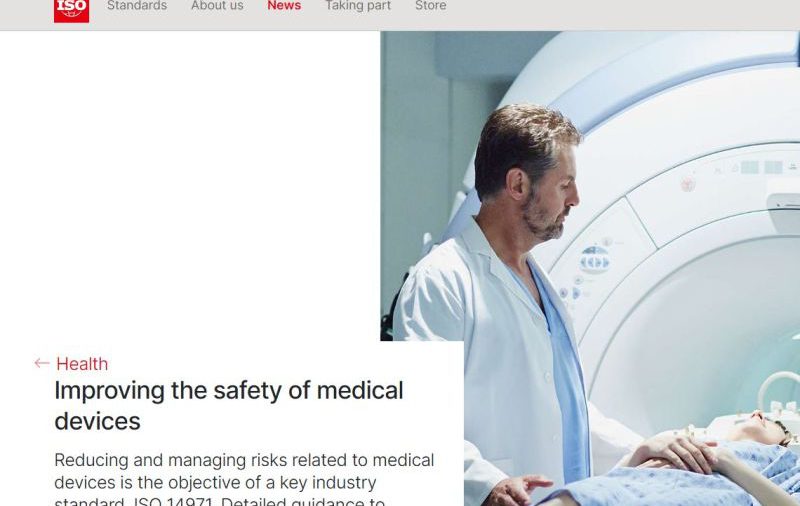
Did you know ISO/TR 24971, Medical devices – Guidance on the application of ISO 14971, is a companion document to the globally recognized risk management standard ISO 14971, Medical devices – Application of risk management to medical devices.
The ISO standard, which was updated in December 2019, specifies the terminology, principles and process for managing the risks associated with medical devices, including software as a medical device (SaMD) and in vitro diagnostic (IVD) medical products.
Providing detailed guidance on how to use the standard most effectively, the recently published technical report – ISO/TR 24971 – clarifies the requirements of the standard and contains recommendations and practical examples on how to achieve compliance with those requirements. It follows the same structure and the same clause numbering as ISO 14971:2019 to facilitate its use.