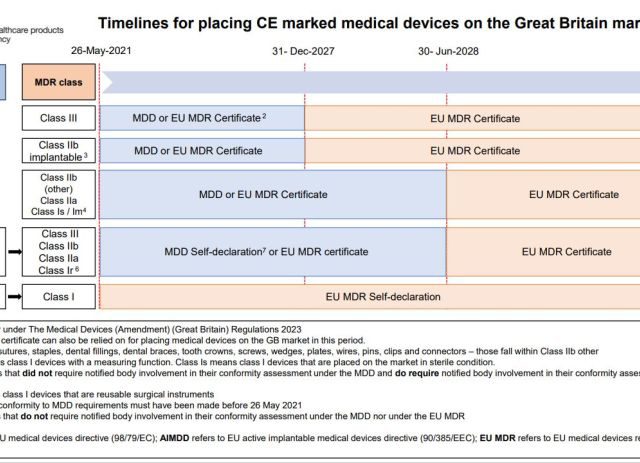
On 18 July, the European Commission published in the Official Journal of the European Union an updated guideline made up of Questions and Answers on the practical aspects related to the implementation of Regulation as they relate to the SCOPE OF THE EXTENSION OF THE…